Antibody Therapeutics Market Overview
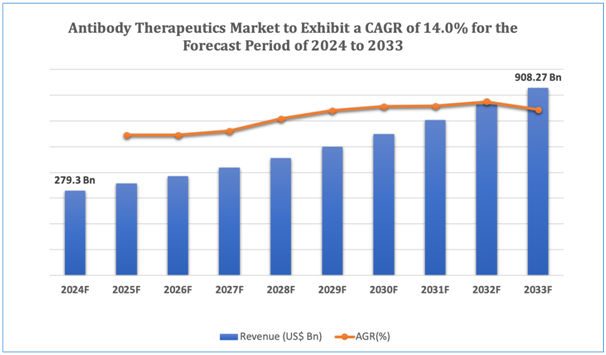
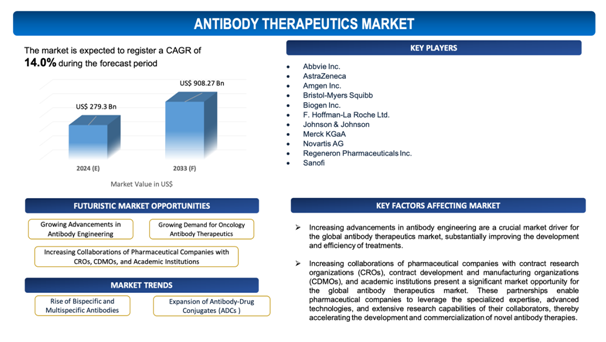
The global Antibody Therapeutics market is estimated to be worth over USD 908.27 Bn in 2033 and is expected to grow at CAGR of 14.0% during the forecast period (2024-2033).
Antibody therapeutics portrays a revolutionary force in the pharmaceutical sector, transforming the treatment panorama for different diseases, comprising autoimmune disorders, infectious diseases, cancer, and more. These therapies includes the use of monoclonal antibodies (mAbs) that are designed to target specific antigens, improving the accuracy and efficacy of treatments. The emergence of antibody therapeutics has offered a means to address previously challenging medical conditions with higher specificity and fewer side effects compared to traditional small-molecule drugs.
One of the essential benefits of antibody therapeutics is their capability to precisely target disease-causing agents without affecting healthy cells. This targeted approach minimizes collateral damage to the body's normal tissues, mitigating adverse effects and enhancing patient outcomes. The development of checkpoint inhibitors, a class of antibody therapeutics, has substantially advanced cancer treatment by releasing the immune system to battletumour cells efficiently. Drugs like nivolumab and pembrolizumab have shown phenomenal success in managing various cancers, comprising melanoma, lung cancer, and more, offering new hope to patients with limited treatment alternatives.
In addition, antibody therapeutics are in the lead of acknowledging the global challenge of infectious diseases. The COVID-19 pandemic highlighted the potential of antibody-based treatments, with therapies such as monoclonal antibodies being deployed to neutralize the virus and minimize disease severity. This has underscored the crucial role of antibody therapeutics in responding to emerging health threats swiftly.
The pharmaceutical industry is experiencing a rise in the development and approval of antibody-based therapies, fuelled by breakthroughs in biotechnology, genetic engineering, and a greater understanding of disease mechanisms. This momentum is further facilitated by regulatory frameworks that prioritize advanced treatments and speed up their approval processes. As a result, antibody therapeutics are not only revolutionizing patient care by providing more effective and safer treatment alternatives but also acceleratingnotable growth and innovation within the pharmaceutical industry, defining the future of medicine.
Figure 1. Antibody Therapeutics: Market Size
Get more details on this report - Request Free Sample
Key Market Insights
The global antibody therapeutics market is witnessingstrong growth, fuelled by growing demand for targeted therapies and developments in biotechnology. Leading market insights reveal that monoclonal antibodies (mAbs) dominate the market, given their efficiency in managing an extensive scale of diseases, comprising cancers, autoimmune disorders, and infectious diseases. The oncology domain, in particular, accounts for the dominating share owing to the increased prevalence of cancer and the success of antibody-based treatments such as checkpoint inhibitors and antibody-drug conjugates.
Key developments underscore the advent of bispecific antibodies, which can concurrently bind to two various antigens, providingimproved therapeutic advantages. In addition, the surge of antibody-drug conjugates (ADCs) combines the particularity of antibodies with the potency of cytotoxic drugs, targeting cancer cells more effectively while sparing healthy tissues. The market is also experiencing the advent of biosimilars, which are poised to offer cost-effective options to existing biologics, thus expanding patient access to these crucial therapies.
Technological innovations in genetic engineering and protein engineering are boosting the development of next-generation antibodies with enhancedefficiency and safety profiles. Advancements such as humanized and fully human antibodies minimize the risk of immunogenicity, improving patient compatibility and treatment outcomes. In addition, developments in single-cell analysis and high-throughput screening are expediting the discovery and optimization of novel antibodies.
The current market landscape is marked by robust investment in research and development, strategic collaborations, and mergers and acquisitions among leading players to improve their product portfolios and market presence. Regulatory bodies are also promoting the market growth by expediting approval processes for advanced antibody therapeutics. All in all, the global antibody therapeutics market is poised for sustained expansion, fuelled by technological developments and a increasing focus on precision medicine.
Market Dynamics
Market Drivers
Growing Advancements in Antibody Engineering
Increasing advancements in antibody engineering are a crucial market driver for the global antibody therapeutics market, substantiallyimproving the development and efficiency of treatments. Antibody engineering comprisescultured techniques to design and optimize antibodies with specific characteristics, such as enhanced binding affinity, improved stability, and minimized immunogenicity. These developmentsallow the creation of highly targeted therapies that can accurately interact with disease-associated antigens, reducing off-target effects and growing therapeutic efficacy.
One of the prominent innovations in antibody engineering is the development of bispecific antibodies, which can concurrently bind to two various antigens or epitopes. This dual-targeting capability providesimproved therapeutic benefits, specifically in oncology, where bispecific antibodies can engage both cancer cells and immune cells to encourage a more robust anti-tumour response. Another major advancement is the creation of antibody-drug conjugates (ADCs), which combine the specificity of antibodies with the potent cytotoxic effects of conventional chemotherapeutic agents, enabling for targeted delivery of drugs to cancer cells while sparing healthy tissues.
Humanization and fully human antibodies represent another breakthrough, as these engineered antibodies minimize the possibility of adverse immune reactions, making them more suitable for long-term use in patients. The emergence of high-throughput screening and next-generation sequencing technologies has also propelled the discovery and optimization process, allowing the swift identification of promising antibody candidates and their subsequent refinement.
These developments in antibody engineering are fuelling market growth by expanding the scale of treatable conditions and enhancing the safety and efficacy profiles of antibody therapeutics. As a result, pharmaceutical companies are more and more investing in research and development to leverage these technologies, leading to a surge in the number of antibody-based therapies entering clinical trials and reaching the market. This trend highlights the transformative impact of antibody engineering on the global antibody therapeutics market, fostering innovation and enhancing patient care.
Market Restraints
With regard to numerous advantages of Antibody Therapeutics, the market faces several challenges due to the unique characteristics and requirements associated with these potent pharmaceutical products. Some of the key market challenges include:
- High Development and Production Costs: The development and manufacturing of antibody therapeutics involve complicated and resource-intensive processes, comprising advanced biotechnological techniques, extensive clinical trials, and stringent regulatory compliance. These increased costs can confine the affordability and accessibility of antibody-based treatments, especially in low- and middle-income regions, thuslimiting market growth.
- Regulatory Challenges and Approval Delays: The regulatory panorama for antibody therapeutics is rigorous, necessitating comprehensive safety and efficacy evaluations before approval. The complex and lengthy approval processes can postpone the advent of new therapies to the market. In addition to that, navigating diverse regulatory requirements across different regions can pose challenges for pharmaceutical companies, potentially hampering the global expansion of antibody therapeutics.
Get more details on this report - Request Free Sample
Market Opportunity
Increasing Collaborations of Pharmaceutical Companies with CROs, CDMOs, and Academic Institutions
Increasing collaborations of pharmaceutical companies with contract research organizations (CROs), contract development and manufacturing organizations (CDMOs), and academic institutions present a significant market opportunity for the global antibody therapeutics market. These partnerships enable pharmaceutical companies to leverage the specialized expertise, advanced technologies, and extensive research capabilities of their collaborators, thereby accelerating the development and commercialization of novel antibody therapies.
Collaborations with CROs enable pharmaceutical companies to outsource different aspects of the drug development process, comprising preclinical studies, clinical trials, and regulatory affairs. This not only minimizes the time and cost related to bringing novel therapies to market but also improves the efficacy and quality of research through access to CROs' specialized knowledge and infrastructure.
In the similar manner, partnering with CDMOs provides pharmaceutical companies with scalable manufacturing solutions and state-of-the-art production facilities. CDMOs provide flexible and cost-effective manufacturing services, from process development to large-scale production, allowing companies to meet the soaring demand for antibody therapeutics without the necessity for significant capital investment in manufacturing infrastructure.
Academic institutions, on the other hand, are centres of innovation and leading-edge research. Collaborations with academic researchers can lead to the discovery of novel antibody targets, the development of advanced therapeutic approaches, and the generation of preclinical data that can be crucial for advancing drug candidates. These partnerships often result in the co-development of ground-breaking therapies and offer pharmaceutical companies with early access to emerging scientific discoveries.
By fostering these strategic collaborations, pharmaceutical companies can improve their research and development capabilities, streamline the drug development process, and expedite the emergence of new antibody therapeutics to the market. This collaborative approach not only fuels innovation but also positions companies to capitalize on the soaring demand for targeted and effective antibody-based treatments, thus presenting a significant market opportunity.
Top of Form
Bottom of Form
Market Trends
- Rise of Bispecific and Multispecific Antibodies: The development and adoption of bispecific and multispecific antibodies are gaining traction in the antibody therapeutics market. These advanced therapies can simultaneously target multiple antigens or epitopes, improving therapeutic efficiency and offering novel treatment alternatives for intricate diseases such as cancer and autoimmune disorders. Their capability to engage multiple pathways or cells in a single treatment makes them a promising and swiftlyincreasing segment within the market.
- Expansion of Antibody-Drug Conjugates (ADCs): Antibody-drug conjugates (ADCs) are becoming progressivelysignificant as a market trend. ADCs combine the targeting capability of monoclonal antibodies with the potent cell-killing capability of cytotoxic drugs, allowingaccurate delivery of chemotherapy directly to cancer cells while minimizing damage to healthy tissues. The success of existing ADC therapies and continuous advancements in ADC technology are boosting significant investment and interest, resulting in the development of a new generation of ADCs with enhancedefficiency and safety profiles.
Antibody Therapeutics Market: Key Segments
By Format
- Monoclonal Antibody
- Polyclonal Antibody Therapy
- Antibody Fragment
- Bispecific Antibody
- Other Antibody Formats
By Disease Area
- Autoimmune & Inflammatory Diseases
- Oncology
- Hematology
- Osteology
- Immunology
- Neurology
- Infectious Diseases
- Other Diseases Areas
By Route of Administration
- Intravenous
- Subcutaneous
- Other Route of Administration
By Source
- Human
- Humanized
- Chimeric
- Other Sources
By End User
- Hospitals
- Long-term Care Facilities
- Other End Users
By Key Geographical Regions
- North America
- Europe
- Asia-Pacific
- Middle East and Africa
- South America
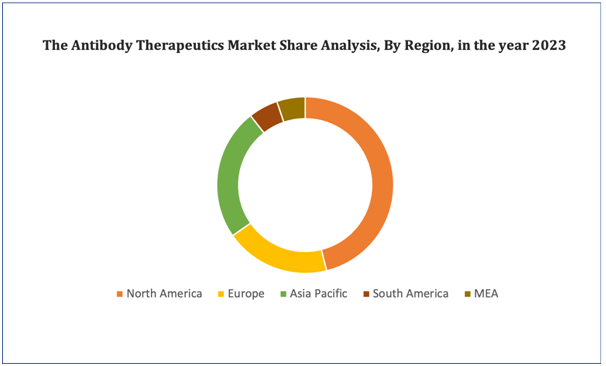
Figure 4. Antibody Therapeutics Market: Distribution by Region
Get more details on this report - Request Free Sample
Antibody Therapeutics Market: Regional Analysis
North America dominates the global antibody therapeutics market attributing to its advanced healthcare infrastructure, notable investment in research and development, and an increased prevalence of chronic diseases requiring innovative treatments. The region boasts a robust biopharmaceutical industry with leading players continuously advancing antibody-based therapies. Regulatory support from agencies like the FDA propels the approval and commercialization of these therapeutics. In addition, substantial funding from government and private sectors fosters innovation and development.
Leading Antibody Therapeutics Developers
Industry Trends and Global Forecasts, 2023-2035 report features an extensive study of the current market landscape, market size and future opportunities associated with the Antibody Therapeuticsmarket, during the given forecast period. Further, the market report highlights the efforts of several stakeholders engaged in this rapidly emerging segment of the biopharmaceutical industry. Key takeaways of the Antibody Therapeuticsmarket are briefly discussed below.
The report includes the list of players operating in the global Antibody Therapeutics market. Some of the key players include:
- Abbvie Inc.
- AstraZeneca
- Amgen Inc.
- Bristol-Myers Squibb
- Biogen Inc.
- F. Hoffman-La Roche Ltd.
- Johnson & Johnson
- Merck KGaA
- Novartis AG
- Regeneron Pharmaceuticals Inc.
- Sanofi
Antibody Therapeutics Market: Key Developments
- In November 2023, Lonza announced today the launch of its new GS Effex® cell line for the development of therapeutic antibodies with enhanced potency.
- In August 2023, Regeneron announces agreement with BARDA supporting development of next-generation antibody therapy for Covid-19 prevention.
Scope of the Report
The market report presents an in-depth analysis of the various firms / organizations that are engaged in this market, across different segments, as defined in the below table:
|
|
Key Report Attributes |
Details |
||
|
|
Base Year |
2023 |
||
|
|
Forecast Period |
2024-2033 |
||
|
|
CAGR (2024-2033) |
14.0% |
||
|
|
Formats |
|
||
|
|
Disease Areas |
|
||
|
|
Route of Administration |
|
||
|
|
Source |
|
||
|
|
End User |
|
||
|
|
Key Geographical Regions |
|
||
|
Key Companies Profiled |
|
|
||
