Hepatitis B Market Overview
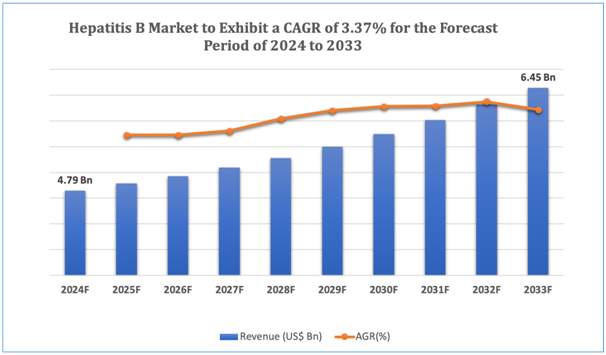
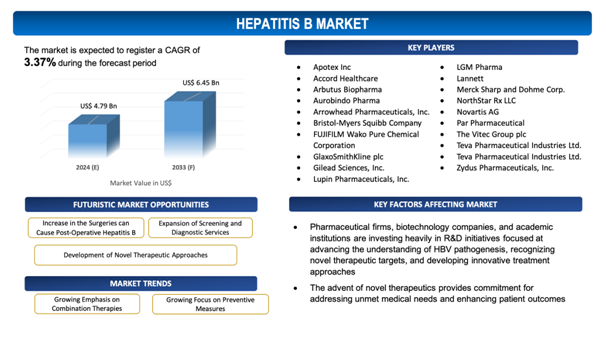
The global Hepatitis B market is estimated to be worth over USD 6.45Bn in 2033 and is expected to grow at CAGR of 3.37% during the forecast period (2024-2033). Hepatitis B stands as a viral infection that affects the liver, induced by the hepatitis B virus (HBV). It is a significant global health concern, with approximately 257 million people living with chronic HBV infection across the world. Hepatitis B can pave its way to severe liver complexities, comprising liver cirrhosis and hepatocellular carcinoma (liver cancer), and is responsible for an approximate 887,000 fatalities annually. The global market for hepatitis B facilitatesvaried sectors, comprising diagnostics, therapeutics, and preventive measures, focused at addressing the burden of HBV infection and its related complications. Diagnostics hold as essential role in the early detection and monitoring of hepatitis B, with tests such as HBsAg (hepatitis B surface antigen) screening, HBV DNA quantification, and liver function tests assisting in diagnosis and disease management. Therapeutic interventions for hepatitis B comprise antiviral medications, such as nucleos(t)ide analogs and interferon-based therapies, which repress viral replication and mitigate the risk of liver-related intricacies. In addition to that, preventive measures such as vaccination against HBV infection have contributed substantially to mitigating the global burden of hepatitis B. The World Health Organization (WHO) recommends universal hepatitis B vaccination as part of routine childhood immunization programs in countries with increased HBV prevalence. Nevertheless, regardless of these interventions, challenges persist in achieving universal access to hepatitis B testing, treatment, and vaccination, specifically in low- and middle-income countries where resources are likely to be limited. In addition, the emergence of drug-resistant HBV strains and gaps in vaccination coverage pose continuing challenges to hepatitis B control efforts globally. Addressing these challenges needs coordinated efforts from governments, healthcare organizations, and industry stakeholders to enhance access to diagnostics, ensure affordable treatment alternatives, and diversify vaccination programs, ultimately curtailing the burden of hepatitis B and its related morbidity and mortality across the world.
Figure 1. Hepatitis B: Market Size
Get more details on this report - Request Free Sample
Key Market Insights &Current Market Landscape:
The global hepatitis B market is defined by major insights and a dynamic outlookfueled by numerous factors, comprisinginnovations in diagnostics, therapeutics, and preventive measures. Significant developments in the market comprisesadvancements in hepatitis B diagnostics, such as the adoption of nucleic acid testing (NAT) for HBV DNA quantification, which facilitates sensitive detection of viral load and monitoring of treatment efficiency. In addition to that, novel technologies in therapeutic interventions, such as the development of next-generation antiviral medications with enhancedefficiency and safety profiles, are revolutionizing hepatitis B treatment paradigms.
Emerging approaches, comprising RNA interference (RNAi) therapies and immune modulators, offercommitment for achieving functional cure in chronic HBV infection. In addition to that, preventive measures such as therapeutic vaccines and immune-based therapies are being investigated for their potential to improve immune response and minimize the risk of HBV transmission and disease progression. Regardless of these advancements, challenges continue in achieving universal access to hepatitis B testing, treatment, and vaccination, specifically in resource-limited settings. Continued efforts in research, innovation, and collaboration are crucial to address the evolving needs of patients with hepatitis B and to mitigate the global burden of this viral infection.
Market Dynamics
Market Drivers
Increasing Research and Development Efforts
With more or less 257 million people living with chronic hepatitis B virus (HBV) infection across the world, there is an urgent need for advanced diagnostics, therapeutics, and preventive measures to tackle the burden of this viral infection and its related complications. Pharmaceutical firms, biotechnology companies, and academic institutions are investing heavily in R&D initiatives focused at advancing the understanding of HBV pathogenesis, recognizing novel therapeutic targets, and developing innovative treatment approaches. These efforts have paved its way to significant advancements in hepatitis B diagnostics, comprising the adoption of sensitive nucleic acid testing (NAT) for viral load quantification and monitoring of treatment response. In addition to that, the development of next-generation antiviral medications with enhancedefficiency, safety profiles, and resistance barriers is revolutionizing hepatitis B treatment paradigms. Along with that, continuing research into immune modulators, RNA interference (RNAi) therapies, and therapeutic vaccines offerscommitment for achieving functional cure and mitigating the global burden of chronic HBV infection. By fueling innovation and fostering collaboration between industry stakeholders and healthcare providers, increasing R&D efforts are accelerating advancements in hepatitis B management and contributing to enhanced outcomes for patients affected by this viral disease.
Market Restraints
With regard to numerous advantages of Hepatitis B, the market faces several challenges due to the unique characteristics and requirements associated with them. Some of the key market challenges include:
- High Cost of Treatment: The increased cost associated with hepatitis B treatment, comprising antiviral medications and diagnostic tests, presents a notableconstraint on access to care, specifically in low- and middle-income countries, confining affordability and availability of essential therapies.
- Challenges in Patient Identification and Monitoring: Difficulty in recognizing and monitoring individuals with chronic hepatitis B infection due to asymptomatic presentation and limited access to diagnostic testing in some regions impedes timely diagnosis, treatment initiation, and disease management, contributing to gaps in care and disease progression.
Market Opportunities
Increase in the Surgeries can Cause Post-Operative Hepatitis B
Since millions of individuals across the globe are suffering from chronic hepatitis B virus (HBV) infection, the advent of novel therapeutics providescommitment for addressing unmet medical needs and enhancing patient outcomes. Pharmaceutical firms are investing in research and development efforts to develop next-generation antiviral medications with enhancedefficiency, safety profiles, and resistance barriers, focusing onimproving viral suppression and mitigate the risk of liver-associatedcomplexities. In addition to that, emerging therapeutic modalities, such as RNA interference (RNAi) therapies, immune modulators, and therapeutic vaccines, hold potential for achieving functional cure and long-term viral control in chronic HBV infection. The approval and commercialization of novel drugs expand treatment alternatives for patients, offering alternatives for those with treatment-resistant strains or intolerance to current therapies. In addition to that, higher competition in the market are likely to drive down drug prices, enhancing affordability and accessibility of hepatitis B treatment globally. By addressing the evolving needs of patients and healthcare providers, increasing new drug releases encourage innovation, promote advancements in hepatitis B management, and contribute to curtailing the global burden of chronic HBV infection.
Market Trends
- Growing Emphasis on Combination Therapies: There is a trend towards the adoption of combination therapies in the global hepatitis B market, leveraging the compound effects of multiple antiviral medications or therapeutic modalities to improve viral suppression, mitigate resistance, and enhance treatment outcomes.
- Growing Focus on Preventive Measures: There is a soaring focus on preventive measures, comprising vaccination programs and screening initiatives, focused at mitigating the incidence of hepatitis B virus (HBV) infection and transmission, specifically in high-risk populations and regions with escalated disease burden.
Get more details on this report - Request Free Sample
Hepatitis B Market: Key Segments
By Type
- Acute
- Chronic
By Treatment
- Antiviral Drugs
- Vaccine
- Immune Modulator Drugs
- Surgery
By Distribution Channel
- Hospital and Retail Pharmacies
- Online Pharmacies
By Key Geographical Regions
- North America
- Europe
- Asia-Pacific
- Middle East and Africa
- South America
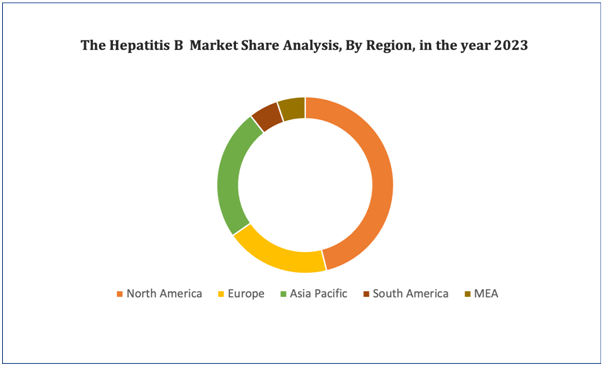
Hepatitis B Market: Regional Analysis
North America dominates the hepatitis B infection market due to increased adoption of hepatitis B infection cases as well as increased healthcare facilities, whereas the Asia-Pacific region is expected to grow at the fastest rate during the forecast period due to significant investment by major companies.
Figure 4. Hepatitis B Market: Distribution by Region
Get more details on this report - Request Free Sample
Leading Hepatitis B Developers
Industry Trends and Global Forecasts, 2023-2035 report features an extensive study of the current market landscape, market size and future opportunities associated with the Hepatitis Bmarket, during the given forecast period. Further, the market report highlights the efforts of several stakeholders engaged in this rapidly emerging segment of the biopharmaceutical industry. Key takeaways of the Hepatitis Bmarket are briefly discussed below.
The report includes the list of players operating in the global Hepatitis Bmarket. Some of the key players include:
- Apotex Inc
- Accord Healthcare
- Arbutus Biopharma
- Aurobindo Pharma
- Arrowhead Pharmaceuticals, Inc.
- Bristol-Myers Squibb Company
- FUJIFILM Wako Pure Chemical Corporation
- GlaxoSmithKline plc
- Gilead Sciences, Inc.
- Lupin Pharmaceuticals, Inc.
- LGM Pharma
- Lannett
- Merck Sharp and Dohme Corp.
- NorthStar Rx LLC
- Novartis AG
- Par Pharmaceutical
- The Vitec Group plc
- Teva Pharmaceutical Industries Ltd.
- Teva Pharmaceutical Industries Ltd.
- Zydus Pharmaceuticals, Inc.
Recent Developments in the Hepatitis B Market
Several recent developments have taken place in the field of Hepatitis B, some of which have been outlined below. These developments, even if they took place post the release of our market report, substantiate the overall market trends that we’ve outlined in our analysis chronologically.
- In February 2024, GSK plc announced that the US Food and Drug Administration (FDA) has granted Fast Track designation for bepirovirsen, an investigational antisense oligonucleotide (ASO) for the treatment of chronic hepatitis B (CHB). Fast track designation is intended to facilitate the development and expedite the review of drugs to treat serious conditions and fill an unmet medical need.
Scope of the Report
The market report presents an in-depth analysis of the various firms / organizations that are engaged in this market, across different segments, as defined in the below table:
|
Key Report Attributes |
Details |
|
Base Year |
2023 |
|
Forecast Period |
2024-2033 |
|
CAGR (2024-2033) |
3.37% |
|
Type |
|
|
Treatment |
|
|
Distribution Channel |
|
|
Key Geographical Regions |
|
|
Key Companies Profiled |
|
