Regenerative MedicineMarket Overview
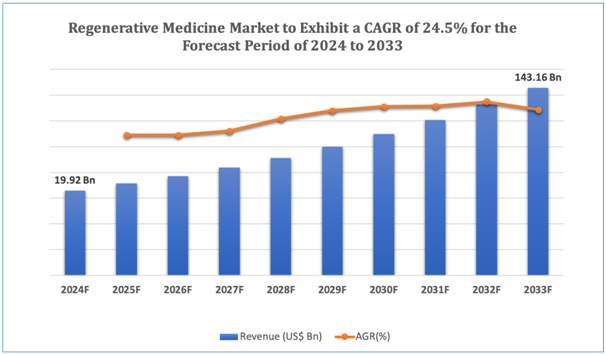
The global regenerative medicine market is estimated to be worth over USD 143.16Bn in 2033 and is expected to grow at CAGR of24.5% during the forecast period (2024-2033).
Regenerative medicine is a swiftly advancing field that emphasizes on repairing, replacing, or regenerating damaged cells, tissues, and organs to restore normal function. This ground-breaking branch of medicine utilizes different approaches, comprising stem cell therapy, tissue engineering, and the use of biomaterials, to support the body's natural healing processes. Regenerative medicine holds the potential to revolutionize the treatment of several conditions, from chronic diseases to acute injuries, offering solutions where conventional therapies may fall short.
One of the most favourable aspects of regenerative medicine is stem cell therapy. Stem cells have the unparalleled ability to differentiate into different cell types, making them invaluable for regenerating damaged tissues. This approach has reflected potential in managing conditions like spinal cord injuries, heart disease, and neurodegenerative disorders. Tissue engineering, another major component, involves creating bioartificial tissues and organs in the lab, which can be used for transplantation or to support the body's own repair mechanisms. Advances in 3D bioprinting are boosting this field, allowing the accurate fabrication of complex tissue structures.
Regenerative medicine is also transforming the panorama by offering personalized treatment options. For instance, induced pluripotent stem cells (iPSCs) can be derived from a patient's own cells, mitigating the risk of immune rejection and tailoring therapies to individual genetic profiles. In addition, the use of biomaterials and growth factors to create supportive scaffolds and environments for tissue regeneration is enhancing the efficacy of these treatments.
The revolutionary impact of regenerative medicine extends beyond clinical applications. It is driving significant changes in medical research, pharmaceutical development, and healthcare delivery. By emphasizing on the underlying causes of diseases rather than merely addressing symptoms, regenerative medicine promises to improve patient outcomes, reduce healthcare costs, and lead the way for innovative therapeutic strategies. As research and technology continue to advance, regenerative medicine is poised to hold a critical role in the future of healthcare.
Figure 1. Regenerative Medicine: Market Size
Get more details on this report - Request Free Sample
Key Market Insights
The global regenerative medicine market is witnessing robust growth, fuelled by advancements in stem cell therapy, tissue engineering, and gene editing technologies. Latest market insights relfect a rise in research funding and strategic partnerships among biotech firms, academic institutions, and healthcare providers, propelling the development and commercialization of regenerative therapies. The market is also benefiting from an increasing aging population and growing prevalence of chronic diseases, which highlight the demand for innovative treatment options.
Significant developments comprisenotable progress in stem cell research, especially the use of induced pluripotent stem cells (iPSCs) for personalized medicine. iPSCs, derived from a patient’s own cells, offer customized therapeutic solutions with reduced risk of immune rejection. Tissue engineering has also seen notable breakthroughs, with 3D bioprinting emerging as a revolutionary technology. This technique allows the precise fabrication of complex tissue structures, advancing the creation of bioartificial organs and tissues for transplantation.
Gene editing technologies, particularly CRISPR-Cas9, are transforming the field by allowing precise modifications to DNA, offering potential cures for genetic disorders and improving the efficiency of regenerative therapies. The integration of biomaterials and growth factors into scaffolds to promote tissue regeneration is further improving treatment outcomes.
The current market landscape is marked by a dynamic and competitive environment, with leading players focusing on clinical trials and regulatory approvals to bring novel therapies to market. North America leads in market share owing to robust R&D infrastructure and supportive regulatory frameworks, while Asia-Pacific is swiftly emerging as a significant market due to increasing investments and favourable government initiatives. Therefore, the regenerative medicine market is poised for sustained expansion, fuelled by technological developments and a soaring focus on personalized and regenerative healthcare solutions.
Market Dynamics
Market Drivers
Focus on Personalized Medicine
The focus on personalized medicine is a major market driver for the global regenerative medicine market, as it leverages the specialized genetic, environmental, and lifestyle factors of individuals to develop personalized therapies. Personalized medicine in regenerative medicine primarily utilizes advanced technologies like induced pluripotent stem cells (iPSCs) and gene editing tools such as CRISPR-Cas9 to create customized treatments. iPSCs, obtained from a patient's own cells, can be reprogrammed to develop into different cell types, providing a highly personalized approach to replace or repair damaged tissues and organs, with minimum risk of immune rejection.
Gene editing technologies allowaccurate modifications at the genetic level, enabling the correction of genetic defects that cause diseases, thus providing targeted and effective treatments. This personalization improves the efficiency of regenerative therapies, enhances patient outcomes, and minimizes adverse effects, making treatments more acceptable and successful.
The move towards personalized medicine is also supported by developments in diagnostic technologies, which promote a greater understanding of an individual's genetic makeup and disease mechanisms. This knowledge enables for the development of highly specific and effective regenerative treatments.
Alongside, the growing demand for personalized therapies is boosting investments and research in regenerative medicine, stimulating the development of novel solutions tailored to individual patient needs. Regulatory bodies are also recognizing the potential of personalized medicine, resulting in faster approvals and support for innovative treatments.
Therefore, the focus on personalized medicine is spurring the growth of the regenerative medicine market by ensuring treatments are more safer,effective, and tailored to the unique needs of each patient.
Market Restraints
With regard to numerous advantages of regenerative medicine , the market faces several challenges due to the unique characteristics and requirements associated with these potent pharmaceutical products. Some of the key market challenges include:
- High Cost of Research and Development: The development of regenerative medicine therapies comprisesnotable investment in research, clinical trials, and advanced technologies. These increased costs can be a significant barrier for smaller companies and start-ups, hindering their ability to compete and innovate in the market. In addition, the expenses associated with the manufacturing and commercialization of these therapies can lead to high treatment costs for patients, potentially deterring widespread adoption and access.
- Regulatory and Ethical Challenges: The regenerative medicine market witnesses stringent regulatory requirements and ethical considerations, especially concerning stem cell research and gene editing technologies. Obtaining regulatory approvals for new therapies can be a lengthy and complex process, postponing market entry and growing development costs. Ethical concerns regarding the use of embryonic stem cells and genetic modifications also pose challenges, potentially resulting in public resistance and restrictive policies that can hamper research and development efforts.

Get more details on this report - Request Free Sample
Market Opportunity
Harnessing the Potential of 3D Printing
Harnessing the potential of 3D printing represents a significant market opportunity for the global regenerative medicine market by revolutionizing the way tissues and organs are fabricated and transplanted. 3D bioprinting technology enables the precise layer-by-layer construction of complex tissue structures using bio-inks composed of living cells and biocompatible materials. This advancementsenables for the creation of customized, patient-specific tissues and organs that can mimic the natural architecture and function of human tissues.
One of the primary benefits of 3D printing in regenerative medicine is its capability to produce complex tissue constructs with high precision, which is essential for replicating the intricate structures found in the human body. This capability facilitates the development of more effective and reliable regenerative therapies. For instance, 3D-printed scaffolds can be designed to facilitate cell growth and tissue regeneration, enhancing the body's natural healing processes. Additionally, this technology can address the shortage of organ donors by creating bioartificial organs tailored to individual patients, potentially reducing transplant rejection rates and improving patient outcomes.
Additionally, 3D printing offers the flexibility to rapidly prototype and test new designs, accelerating the pace of innovation and reducing the time and cost associated with developing regenerative therapies. The customization aspect of 3D printing aligns with the growing trend towards personalized medicine, as it allows for the creation of bespoke solutions tailored to the unique anatomical and pathological needs of each patient.
Overall, the incorporation of 3D printing technology in regenerative medicine presents a transformative opportunity to improve the precision, effectiveness, and accessibility of regenerative therapies, driving growth and innovation in the market.
Top of Form
Bottom of Form
Market Trends
- Advancements in Gene Editing Technologies: The global regenerative medicine market is experiencingnotable advancements in gene editing technologies, especially with the widespread adoption of CRISPR-Cas9. This technology allowsaccurate, targeted modifications to the genome, providing the potential to correct genetic defects and manage an extensive range of genetic disorders. The capability to edit genes with high accuracy is fuelling research and development in regenerative therapies, resulting inadvanced treatments that can address previously untreatable conditions and improve the effectiveness of regenerative medicine.
- Increased Focus on Stem Cell Research and Therapies: There is a soaring focus on the development and application of stem cell therapies within the regenerative medicine market. Stem cells, particularly induced pluripotent stem cells (iPSCs) and mesenchymal stem cells (MSCs), are being extensively researched for their potential to regenerate damaged tissues and organs. This trend is facilitated by significant investments in stem cell research and increasing clinical trials focused at validating the efficiency and safety of stem cell-based treatments. As a result, new stem cell therapies are emerging, providing promising solutions for conditions such as spinal cord injuries, heart diseases, and neurodegenerative disorders, thus accelerating market growth.
Regenerative Medicine Market: Key Segments
By Product
- Cell Therapy
- Stem Cell Therapy
- CellTransplantations
- Stem Cell Therapy Products
- Autologous Therapy
- Allogenic Therapy
- Cell-Based Immunotherapy Products
- Gene Therapy
- Tissue Engineering
By Therapeutic Area
- Oncology
- Musculoskeletal Disorders
- Dermatology & Wound Care
- Cardiovascular Diseases
- Ophthalmology
- Neurology
- Other Applications
By Key Geographical Regions
- North America
- Europe
- Asia-Pacific
- Middle East and Africa
- South America
Figure 4. Regenerative Medicine Market: Distribution by Region
Get more details on this report - Request Free Sample
Regenerative Medicine Market: Regional Analysis
North America holds the dominating position in the global market. The high growth is attributed to the availability of government and private funding for development, the presence of advanced tech frameworks to facilitate the rapid detection of chronic diseases and high healthcare spending in the region. Additionally, several ongoing clinical trials for regenerative medicine by leading market players have contributed to the region’s growth.
Leading Regenerative Medicine Developers
Industry Trends and Global Forecasts, 2023-2035 report features an extensive study of the current market landscape, market size and future opportunities associated with the Regenerative Medicinemarket, during the given forecast period. Further, the market report highlights the efforts of several stakeholders engaged in this rapidly emerging segment of the biopharmaceutical industry. Key takeaways of the Regenerative Medicinemarket are briefly discussed below.
The report includes the list of players operating in the global Regenerative Medicinemarket. Some of the key players include:
- Amgen Inc.
- Biogen Inc.
- CORESTEM Inc.
- Gilead Sciences Inc.
- JCR Pharmaceuticals Co. Ltd.
- Medipost Co. Ltd.
- Novartis AG
- Sarepta Therapeutics Inc.
- Smith+Nephew
- Takeda Pharmaceuticals Company Limited
Regenerative Medicine Market: Key Developments
- In March 2024, Pacira BioSciences, Inc., the industry leader in its commitment to non-opioid pain management and regenerative health solutions, announced that the U.S. Food and Drug Administration (FDA) has granted Regenerative Medicine Advanced Therapy (RMAT) designation to PCRX-201 (enekinragene inzadenovec), the company’s novel, intra-articular helper-dependent adenovirus (HDAd) gene therapy product candidate that codes for interleukin-1 receptor antagonist (IL-1Ra), for the treatment of osteoarthritis of the knee.
- In October 2023, Bayer AG announced the opening of its first Cell Therapy Launch Facility in Berkeley, California to create the capacity to bring cell therapies to patients on a global scale. The $250 million (USD), 100,000-square-foot facility will supply the material required for late-stage clinical trials and potential commercial launch of BlueRock Therapeutics’ bemdaneprocel (BRT-DA01), an investigational cell therapy currently in evaluation for treating Parkinson’s disease.
Scope of the Report
The market report presents an in-depth analysis of the various firms / organizations that are engaged in this market, across different segments, as defined in the below table:
|
|
Key Report Attributes |
Details |
||
|
|
Base Year |
2023 |
||
|
|
Forecast Period |
2024-2033 |
||
|
|
CAGR (2024-2033) |
24.5% |
||
|
|
Product |
|
||
|
|
Therapeutic Area |
|
||
|
|
Key Geographical Regions |
|
||
|
Key Companies Profiled |
|
|
||
