Severe Asthma Market Overview
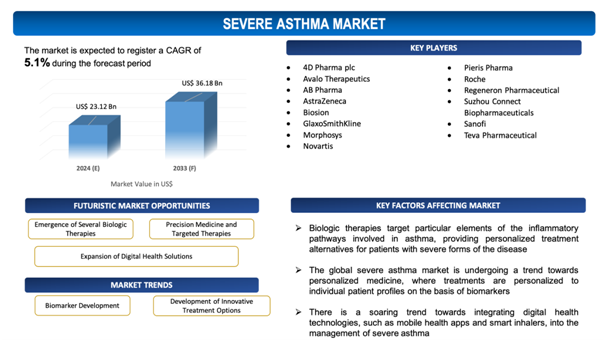
The global Severe Asthma market is estimated to be worth over USD 36.18Bn in 2033 and is expected to grow at CAGR of 5.1% during the forecast period (2024-2033).
Severe asthma portrays as a challengingsubgroup of asthma marked by consistent symptoms, frequent aggravations, and poor response to standard treatments. It affects a considerable portion of the asthma population, contributing to significant mortality, morbidity, and healthcare costs across the globe. Contrary to the mild to moderate asthma, severe asthma oftentimesrequires high-dose corticosteroids or other systemic medications to achieve symptom control, putting patients at risk of side effects and long-term complexities. In addition to that, severe asthma poses considerable burdens on patients' quality of life, resultingin limitations in routine activities, escalated healthcare utilization, and diminished productivity.
The global market for severe asthma comprisesdifferent diagnostic tools, treatment modalities, and supportive care interventions emphasized at enhancing patient outcomes and addressing unmet medical needs. Significant developments in the market comprise advancements in understanding the underlying inflammatory pathways and phenotypes fueling severe asthma, paving its way to the determination of novel therapeutic targets. Biologic therapies targeting specific inflammatory mediators, such as interleukin-5 (IL-5) or immunoglobulin E (IgE), have surfaced as promising treatment alternatives for severe asthma patients with eosinophilic or allergic phenotypes. These biologics provide targeted suppression of inflammatory pathways, mitigating exacerbation rates, enhancing lung function, and improving patients' overall quality of life.
Along with that, precision medicine approaches and biomarker testing hold an integral role in guiding treatment decisions and recognizing patients who are most likely to benefit from specific therapies. Biomarkers such as blood eosinophil counts, fractional exhaled nitric oxide (FeNO), and serum periostin levels help determine patients with eosinophilic inflammation or type 2 inflammation, supporting personalized treatment strategies tailored to individual patient characteristics and disease phenotypes.
Regardless of these advancements, challenges continue in the management of severe asthma, comprising treatment resistance, exacerbation risk, and access to specialized care. However, with continuing research, innovation, and collaboration across healthcare sectors, the global market for severe asthma continues to evolve, offering commitment for enhanced symptom control, curtailed disease burden, and enhanced quality of life for individuals affected by this complex and debilitating respiratory condition.
Figure 1. Severe Asthma: Market Size

Get more details on this report - Request Free Sample
Key Market Insights &Current Market Landscape:
The global severe asthma market is experiencing significant developments and innovations in its current panorama. Severe asthma, marked by persistent symptoms and poor response to standard treatments, represents a significant unmet medical need, fueling research and innovation in the domain. Substantial developments comprise a greater understanding of the underlying inflammatory pathways and phenotypes propelling severe asthma, resulting in the identification of novel therapeutic targets. Biologic therapies, especially monoclonal antibodies targeting specific inflammatory mediators such as interleukin-5 (IL-5) and immunoglobulin E (IgE), have appeared as promising treatment alternatives for severe asthma patients with eosinophilic or allergic phenotypes. These biologics provide targeted suppression of inflammatory pathways, leadingto reduced exacerbation rates, enhanced lung function, and improved quality of life for patients.
Along with that, breakthroughs in precision medicine approaches and biomarker testing have transformed the management of severe asthma, enabling for personalized treatment strategies personalized to individual patient characteristics and disease phenotypes. Biomarkers such as blood eosinophil counts, fractional exhaled nitric oxide (FeNO), and serum periostin levels aiddetermine patients with specific inflammatory profiles, guiding treatment decisions and optimizing therapeutic outcomes.
In addition to that, the advent of novel technologies, including advanced imaging modalities, telemedicine, and digital health platforms, has expanded access to specialized care and enhanced patient monitoring and management. These technologies enable healthcare providers to remotely assess disease status, track treatment response, and offer personalized interventions, ultimately improving patient care and outcomes in the global severe asthma market. Notwithstanding with the challenges such as treatment resistance and healthcare disparities, continuous research, innovation, and collaboration across healthcare sectors continue to fuel progress and transformation in the management of severe asthma, providing promise for enhanced symptom control and reduced disease burden for patients across the world.
Market Dynamics
Market Drivers
Increasing Prevalence of Severe Asthma
Severe asthma represents a significantstrain on healthcare systems across the world, with a soaring number of individuals affected by frequent exacerbations, persistent symptoms, and poor treatment responsiveness. Factors such as environmental pollution, urbanization, allergen exposure, and lifestyle changes contribute to the increasing prevalence of severe asthma across the world. Since the incidence of asthma, especially severe forms, persists to rise, there is a corresponding rise in demand for diagnostic tools, treatment alternatives, and supportive care interventions to address the unmet medical necessities of affected individuals. This growing prevalence not only underlines the pressing need for effective management strategies but also fuels investment in research and development efforts focused at advancing understanding of the underlying mechanisms of severe asthma and determining novel therapeutic targets. Pharmaceutical firms, biotechnology companies, and healthcare providers are increasingly emphasized on innovating and expanding the armamentarium of treatment alternatives available for severe asthma patients, focusing on improving symptom control, mitigate exacerbation rates, and improve the overall quality of life for individuals affected by this exhausting respiratory condition.
Market Restraints
With regard to numerous advantages of Severe Asthma, the market faces several challenges due to the unique characteristics and requirements associated with them. Some of the key market challenges include:
- High Cost of Treatment: Biologic therapies and advanced treatment alternatives for severe asthma oftentimes come with increased price tags, confining accessibility for patients and draining healthcare budgets. The financial strainrelated to these treatments can be a substantial barrier to widespread adoption and patient adherence, specifically in low- and middle-income countries.
- Limited Awareness and Diagnosis: There is a substantial gap in the awareness and proper diagnosis of severe asthma among healthcare professionals and patients alike. This inadequacy of recognition can delay appropriate treatment interventions, resulting in suboptimal management of the condition. The difficulty of diagnosing severe asthma, which time and againneeds specialized tests and expertise, further aggravates this issue, hampering market growth and the effective utilization of available treatments.
Market Opportunities
Emergence of Several Biologic Therapies for Severe Asthma
Biologic therapies target particular elements of the inflammatory pathways involved in asthma, providing personalized treatment alternatives for patients with severe forms of the disease. These therapies, such as monoclonal antibodies targeting interleukins (IL-5, IL-4, IL-13) and immunoglobulin E (IgE), have reflected effectiveness in mitigating exacerbations, enhancing lung function, and improving the quality of life for patients with eosinophilic or allergic asthma phenotypes. The emergence of biologics tackles a crucial unmet need in the treatment outlook, catering to patients who are unresponsive to traditional corticosteroids and long-acting beta-agonists. Since the prevalence of severe asthma increases and the understanding of its underlying biological mechanisms expands, the demand for these targeted therapies is estimated to rise. This trend not only opens new horizons for pharmaceutical firms to develop and market advanced treatments but also stimulates investment in research and development, ultimately broadening the therapeutic options available for severe asthma. As a result, the advent and adoption of biologic therapies are poised to accelerate market growth, providing new commitment for patients and reshaping the management of severe asthma.
Market Trends
- Personalized Medicine and Biomarker Development: The global severe asthma market is undergoing a trend towards personalized medicine, where treatments are personalized to individual patient profiles on the basis of biomarkers. Advances in understanding the heterogeneity of asthma have paved its way to the identification of specific phenotypes and endotypes, driving the use of biomarkers to guide therapy choices. This approach optimizes treatment efficacy, mitigates side effects, and enhances patient outcomes.
- Integration of Digital Health Technologies: There is a soaring trend towards integrating digital health technologies, such as mobile health appsand smart inhalers, into the management of severe asthma. These technologies improve patient monitoring, adherence to treatment plans, and facilitate real-time data collection about disease control and medication use. The adoption of digital tools is enhancing the personalized management of asthma, promoting better communication between patients and healthcare providers, and enabling more precise adjustments to treatment regimens.
Get more details on this report - Request Free Sample
Severe Asthma Market: Key Segments
By Drug Class
- Reslizumab
- Benralizumab
- Anti-inflammatory
By Route of Administration
- Oral
- Inhaled
- Intravenous
- Subcutaneous
By Device Type
- Dry powder inhalers
- Metered dose inhalers
- Soft mist inhalers
By Key Geographical Regions
- North America
- Europe
- Asia-Pacific
- Middle East and Africa
- South America
Severe Asthma Market: Regional Analysis
The North America severe asthma treatment market is expected to grow significantly in the coming years due to several factors, including the high prevalence of asthma in the region, increasing awareness and diagnosis of severe asthma, and advancements in treatment options.In the United States, an estimated 25 Billion people have asthma, and approximately 5-10% of these individuals have severe asthma that is difficult to control with conventional treatments. This represents a significant patient population that is in need of specialized care and effective treatment options.
Figure 4. Severe Asthma Market: Distribution by Region

Get more details on this report - Request Free Sample
Leading Severe Asthma Developers
Industry Trends and Global Forecasts, 2023-2035 report features an extensive study of the current market landscape, market size and future opportunities associated with the Severe Asthmamarket, during the given forecast period. Further, the market report highlights the efforts of several stakeholders engaged in this rapidly emerging segment of the biopharmaceutical industry. Key takeaways of the Severe Asthmamarket are briefly discussed below.
The report includes the list of players operating in the global Severe Asthmamarket. Some of the key players include:
- 4D Pharma plc
- Avalo Therapeutics
- AB Pharma
- AstraZeneca
- Biosion
- GlaxoSmithKline
- Morphosys
- Novartis
- Pieris Pharma
- Roche
- Regeneron Pharmaceutical
- Suzhou Connect Biopharmaceuticals
- Sanofi
- Teva Pharmaceutical
Recent Developments in the Severe Asthma Market
Several recent developments have taken place in the field of Severe Asthma, some of which have been outlined below. These developments, even if they took place post the release of our market report, substantiate the overall market trends that we’ve outlined in our analysis chronologically.
- In September 2022, AstraZeneca’s Tezspire (tezepelumab) has been approved in the European Union (EU) as an add-on maintenance treatment in patients 12 years and older with severe asthma who are inadequately controlled with high dose inhaled corticosteroids plus another medicinal product.The approval by the European Commission was based on results from the PATHFINDER clinical trial programme, which included the pivotal NAVIGATOR Phase III trial in which Tezspire demonstrated superiority across every primary and key secondary endpoint in patients with severe asthma, compared to placebo, when added to standard therapy. The approval follows the recommendation by The Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency in July 2022.
Scope of the Report
The market report presents an in-depth analysis of the various firms / organizations that are engaged in this market, across different segments, as defined in the below table:
|
Key Report Attributes |
Details |
|
Base Year |
2023 |
|
Forecast Period |
2024-2033 |
|
CAGR (2024-2033) |
5.1% |
|
Drug Class |
|
|
Route of Administration |
|
|
Device Type |
|
|
Key Geographical Regions |
|
|
Key Companies Profiled |
|
